4n+2 Rule
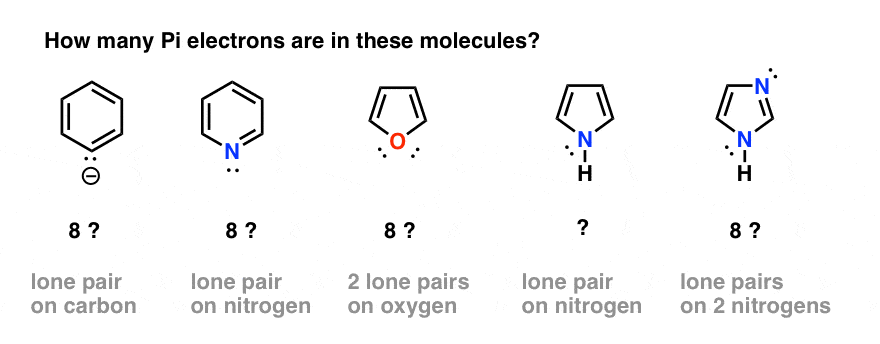
Rules For Aromaticity The 4 Key Factors Master Organic Chemistry

Huckel S Rule Definition Formula And Examples
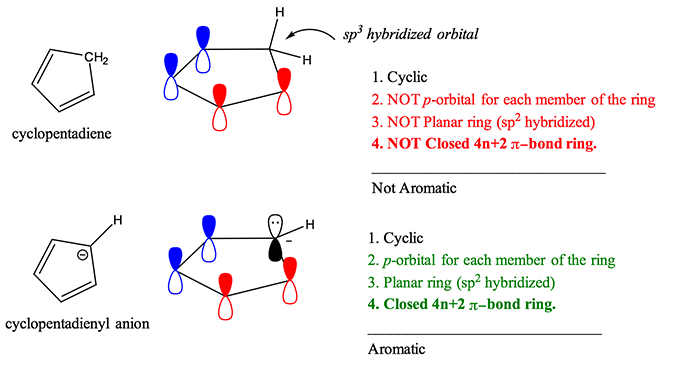
Aromaticity Rules And Definition Organic Chemistry Help
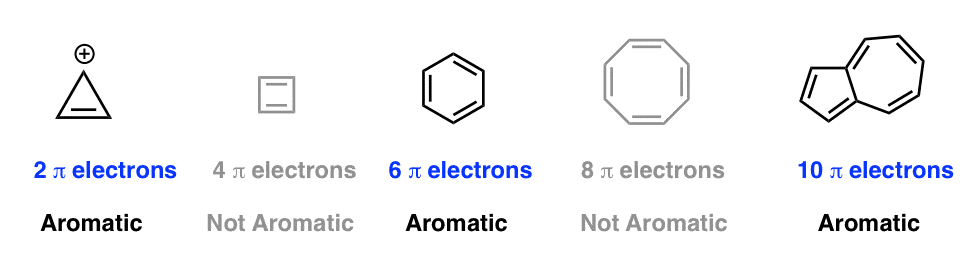
Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry
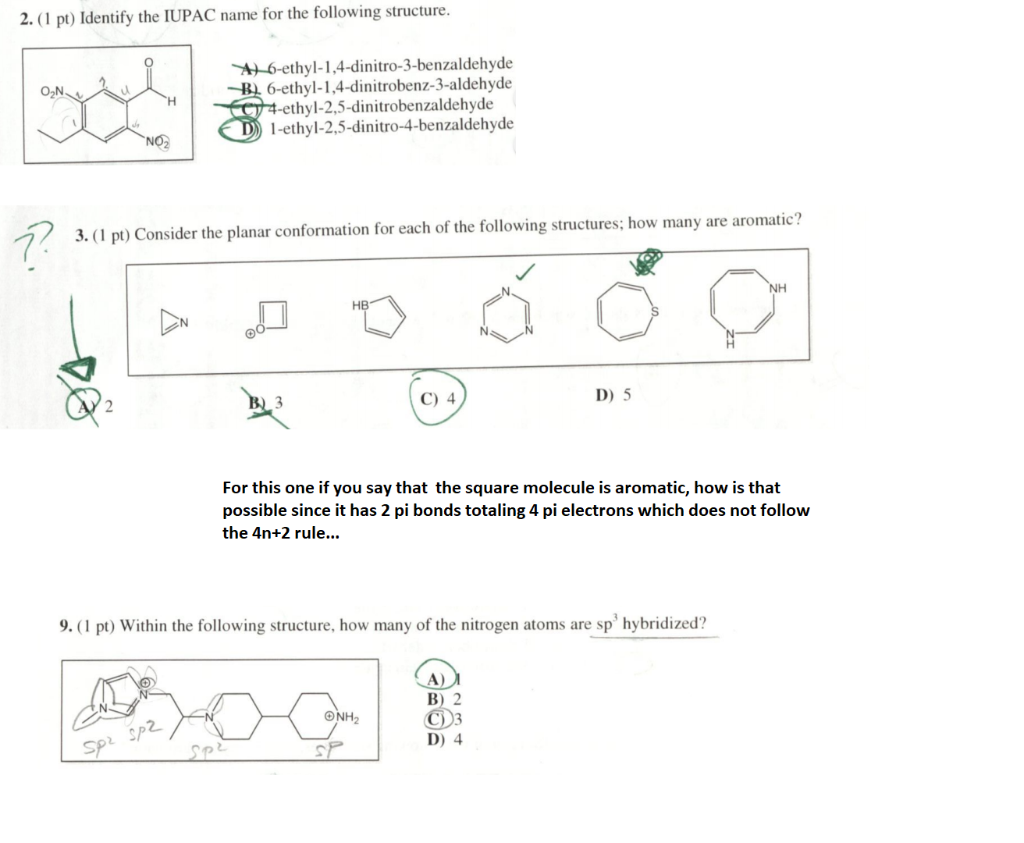
Solved Please Help Me Solve These 3 Questions Also For Chegg Com
Pericyclic Reaction Selection Rules
It is not cyclic.

4n+2 rule. This rule estimates whether a planar ring compound will possess aromatic properties or not. A cyclic ring molecule follows Hückel's rule when the number of its π electrons equals 4n + 2 where n is. It is not planar It does not have an electrons.
A refinement specific to Polycyclic Aromatic Hydrocarbons (PAH) is Clar's rule. Resonating electrons include both pi electrons and lone pairs. The "4n + 2 rule" is derived analytically at the level of the simple Hückel theory for neutral even-membered chains, their double ions, as well as cations and anions of the odd-membered chains, by determining the first order energetic effect of the ring closure.
Furthermore, the 4n+2 rule as indicator of aromatic stabilization should only be used in conjunction with the ring size;. -for some reason none of my organic chemistry textbooks, lectures, etc explain what N actually is, so I made this video. As Huckel formulated, the latex 4n + 2 /latex rule applies only to monocyclic.
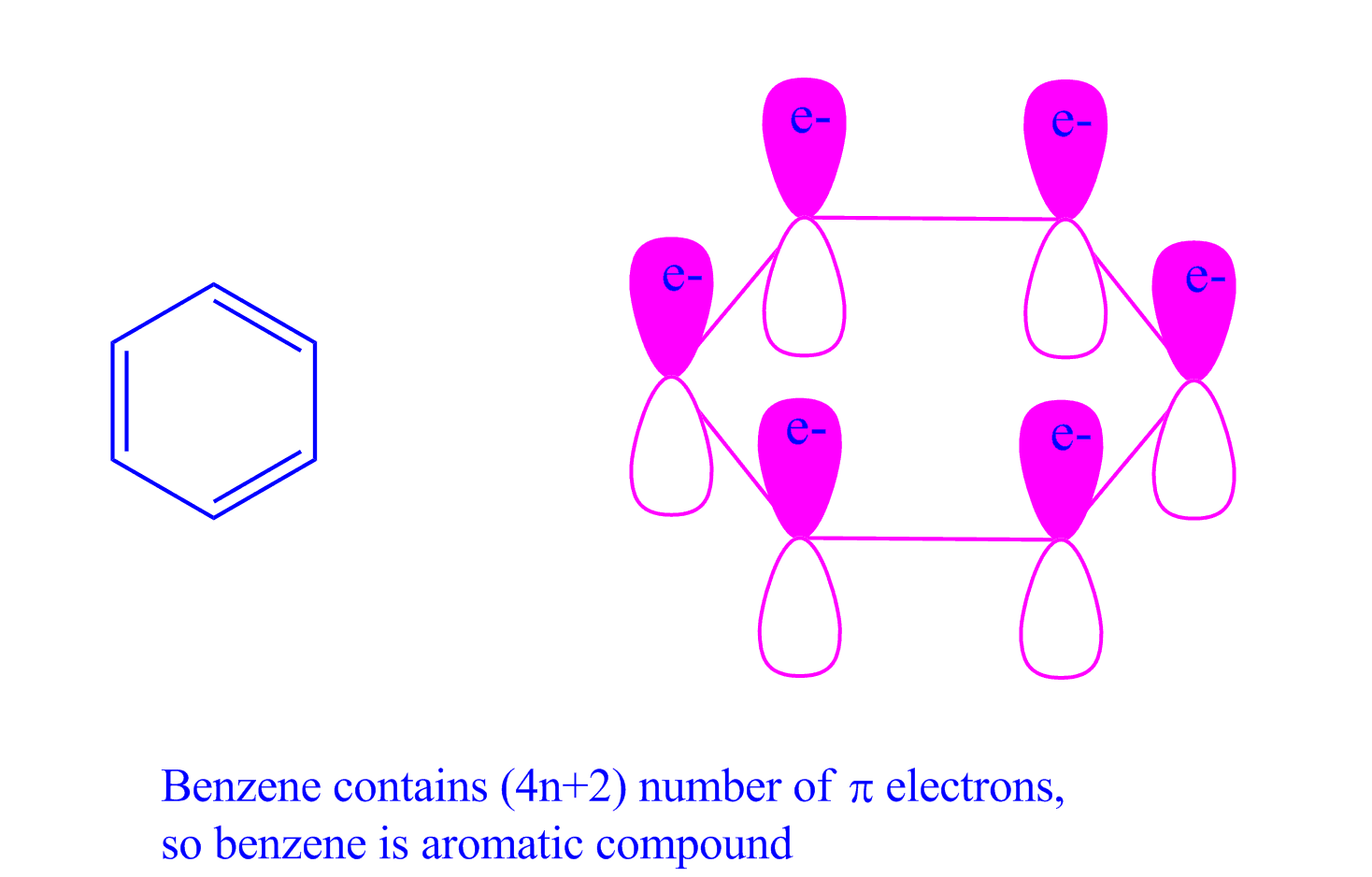
A benzene ring has 3 pi bonds thus 6 resonating. Describe the difference in properties between an aromatic hydrocarbon. Number of times cited according to CrossRef:.
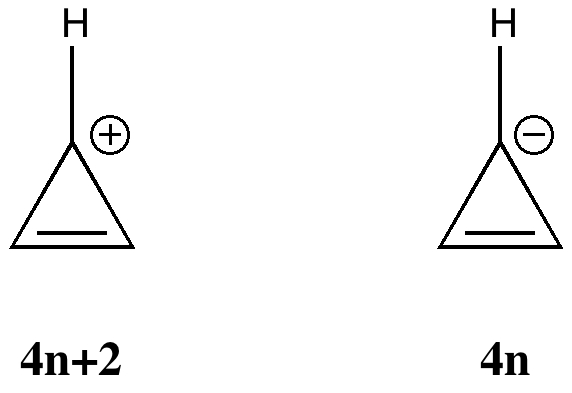
A compound to become aromatic should obey Huckel’s rule. Get 1:1 help now from expert Chemistry tutors. A ring-shaped cyclic molecule is said to follow the Huckel rule when the total number of pi electrons belonging to the molecule can be equated to the formula ‘4n + 2’ where n can be any integer with a positive value (including zero).
Complete delocalization of pie electrons should take place in the atom. This rule would come to be known as Hückel's Rule. Indeed, Hückel's rule can only be theoretically justified for monocyclic systems.
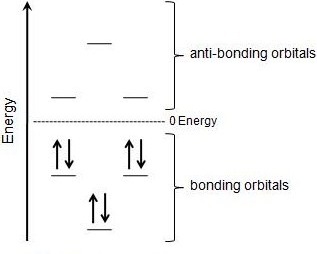
The much greater stabilization in “aromatic” conjugated rings, and Hückel’s 4n+2 rule, derive from alternating stabilization and destabilization of successive orbitals when the ends of a conjugated chain overlap as it is closed to form a ring. Solid working knowledge of hybridization will help you tackle problems of this sort. It was first expressed succinctly as the 4n+2 (actually 2+4n) rule by von Doering in 1951.
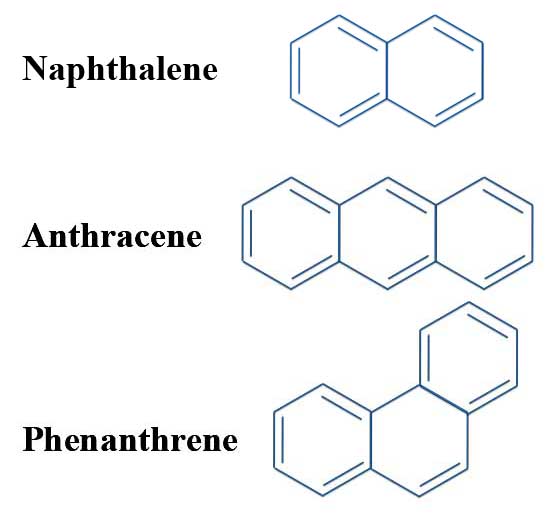
Both of these polycyclic molecules are aromatic even though they fail the 4n + 2 rule. Schlegel diagrams of buckminsterfullerene (top diagram) and four of its isomers which can be built from naphthalene units. Parabola, Finding the Vertex :.
Huckel's Rule (4n+2 rule):. 폰 되링이 1951년 4n+2꼴을 밝혀냈다. Monocyclic planar (or almost planar) systems of trigonally (or sometimes digonally) hybridized atoms that contain (4 n + 2) π-electrons (where n is a non-negative integer) will exhibit.
:o Also, that isn't Osborne's rule itself, but an example where it was applied in the MathWorld link. Before understanding the Huckel’s rule, you need to have a good understanding of aromaticity. In fact OP made no mention of where a description of Osborne's rule could be found, and I had to add it in.
Can 4n+2 equal six, given the restrictions on the value of n?. Therefore, the K(4n + 2) rule is of rather limited range and the table given in Ref. The succinct expression as the 4n + 2 rule has been attributed to W.
When n = 1, for example, there are six pi-electrons, as for benzene. In 1930 Erich Huckel proposed a rule name Huckel’s rule or (4n+2) rule. What is N in the 4n+2 rule?.
It is also known as Huckel’s 4n + 2 pi- electrons rule. Huckel's rule can become a little bit tricky when cations and anions are being evaluated for aromaticity. The Huckel 4n + 2 Pi Electron Rule.
The theory is appropriately called Huckel MO theory, and the rule is Huckel’s latex 4n + 2 /latex rule. The last one is that the organic compound has to be flat. Aromatic compounds contain 4n+2 π electrons, where n is a whole number starting from 0.
The nature of the occupied π orbitals must always be examined. Be planar, in an sp2 hybridized orbital, over every atom of the ring;. This is called the Hückel’s rule discovered by Erich Hückel in 1931.
The 4n+2 rule dats da Huckel's Rule. Huckel’s rule was given by German physicist and physical chemistry Erich Huckel in 1931. The topological background of the "4n + 2 rule" is also briefly discussed.
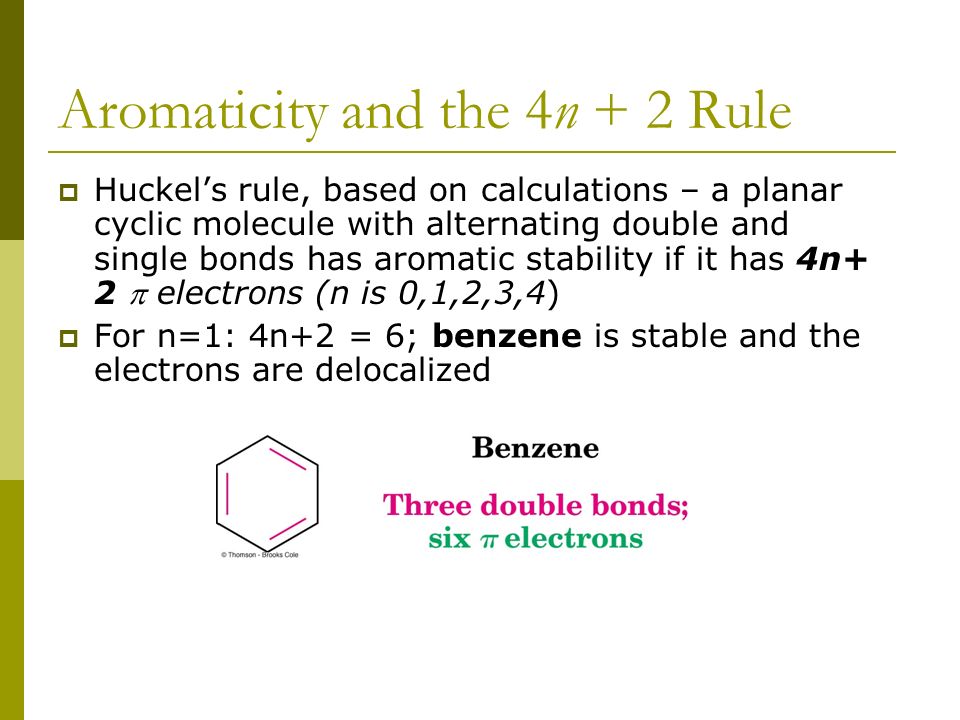
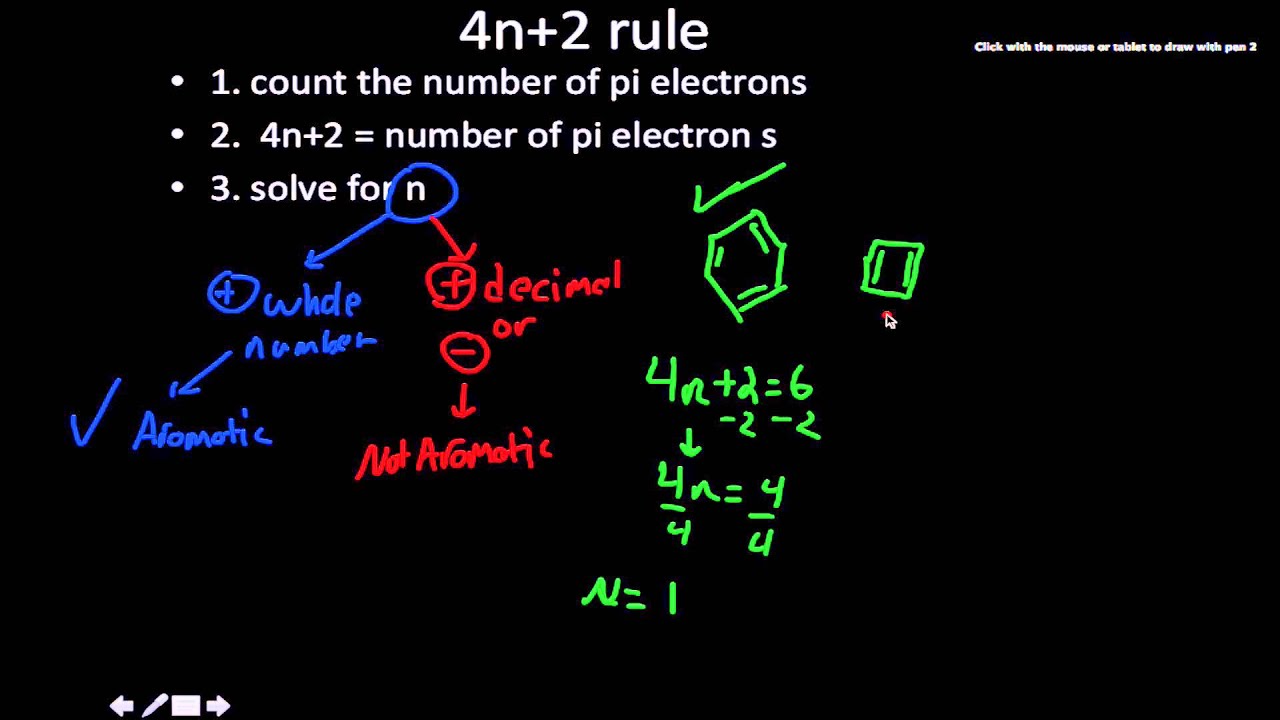
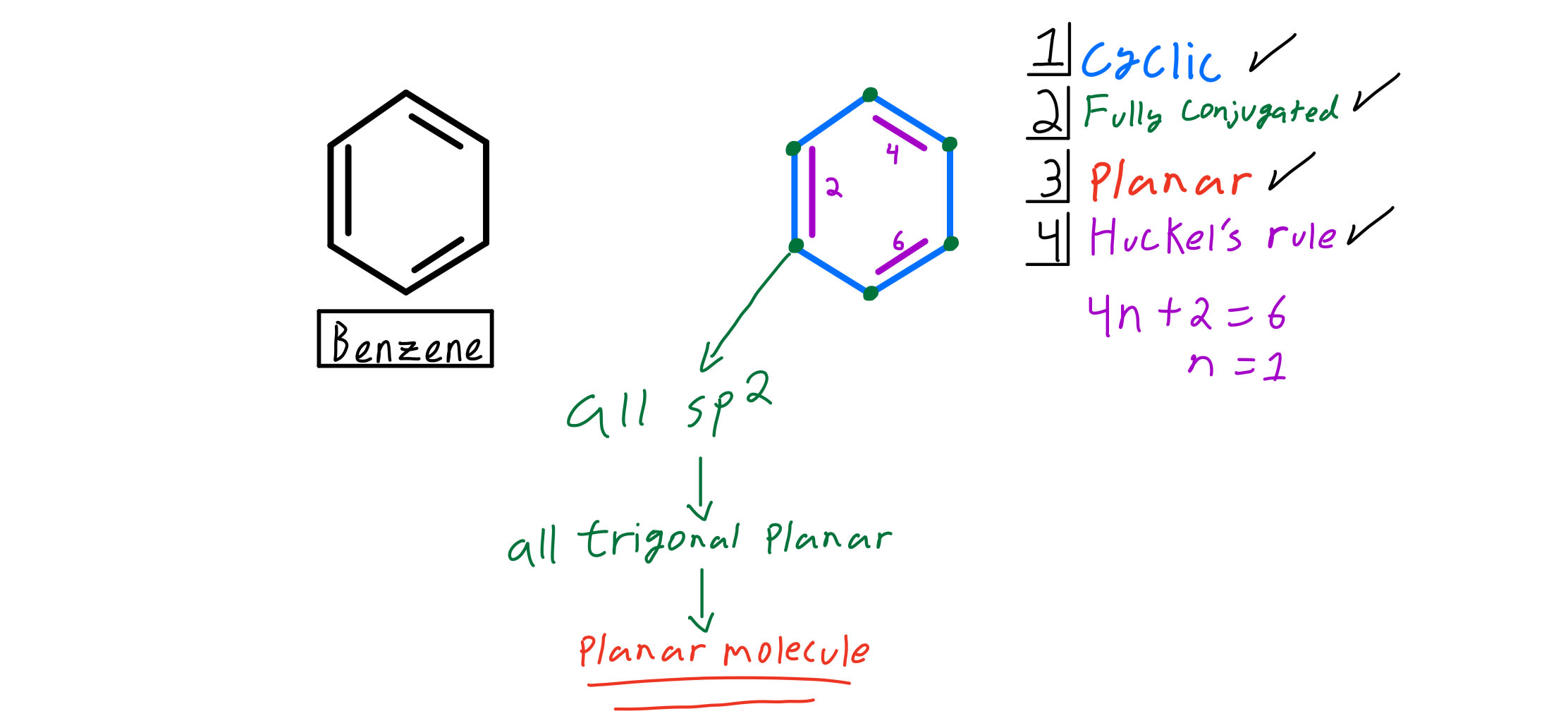
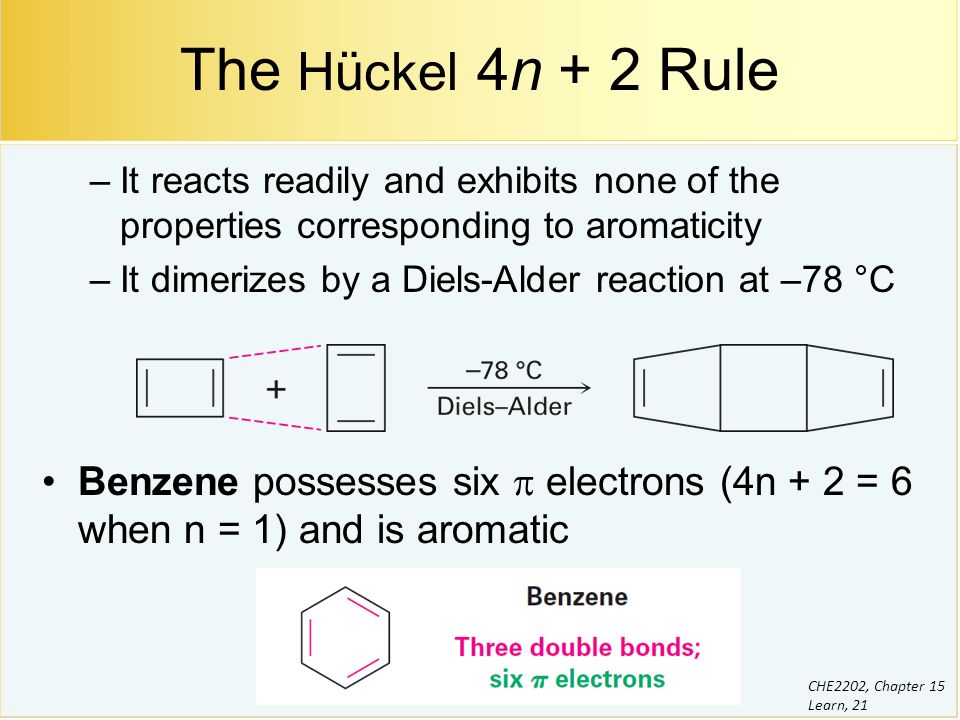
4n + 2 = π 4n + 2 = 6 4n + 2 = 6 4n = 6-2 4n = 4 n = 4/4 n = 1 For benzene, we. This rule is the work of the German theoretician, E. In organic chemistry, Huckel's rule estimates whether a planar ring molecule will have aromatic.
Have a closed loop of 4n+2 pi-bond electrons, where n is equal to any integer (0,1,2,3,…) But like most natural phenomenon, there exists a rule exactly the opposite. It follows the same 4n+2 rule as for benzene, but this time counting the electrons involved in the reaction rather than just the π-electrons in the aromatic ring. Doering (1951), although several authors were using this form at around the same time.
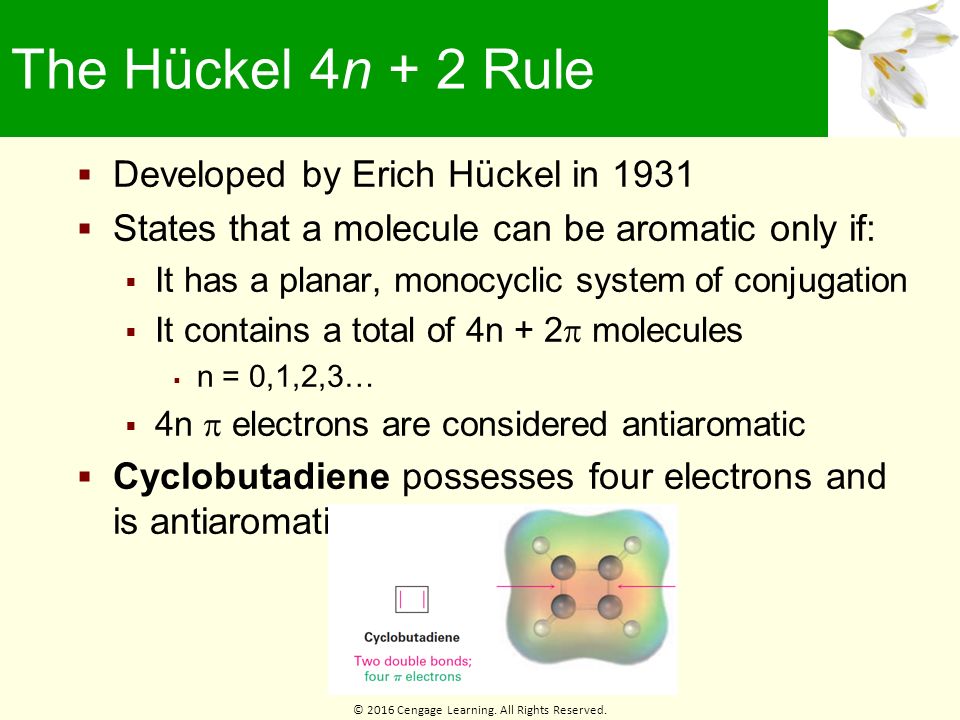
The compound must contain sp2 hybridised carbon atoms. It does not have a cycle of rt bonds. (organic chemistry) Aromatic (ring) compounds must have 4 n + 2 pi-bonding electrons, where n is a whole number and generally limited to n = 0 to 5.
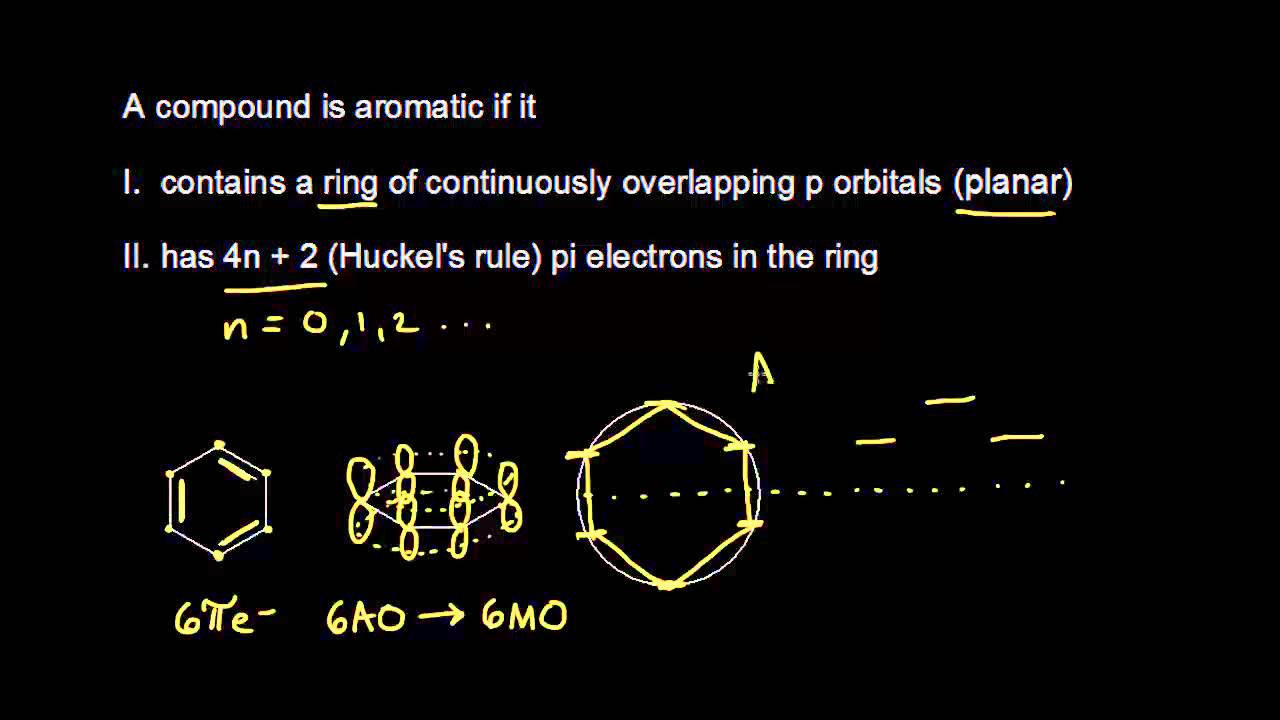
Number of electrons = 4 x 1 + 2 = 6:. If is 0 or any positive integer (1, 2, 3,), the rule has been met.-For example, benzene has six-π electrons:. Hückel's Rule In 1931, Erich Hückel postulated that monocyclic (single ring) planar compounds that contained carbon atoms with unhybridized atomic p orbitals would possess a closed bond shell of delocalized π electrons if the number of π electrons in the molecule fit a value of 4 n + 2 where n equaled any whole number.
Benzene is the most common aromatic molecule. Let us now solve the equation by Completing The Square and by using the Quadratic Formula. Huckel, who devised the simple form of molecular orbital theory we have described in this chapter.
Huckel's rules are a set of few rules to identify an aromatic compound as benzene(C6H6). The “4 n + 2 rule” is derived analytically at the level of the simple Hückel theory for neutral even-membered chains, their double ions, as well as cations and anions of the odd-membered chains, by determining the first order energetic effect of the ring closure. 물리화학자 에리히 휘켈이 1931년에 처음 연구하였다.
Why the structure is so stable is to be explained using resonance structures and the Huckel’s (4n+2) rule. Have one p orbial per atom of the ring;. The rules are as follows:- 1.
The pi electron count is defined by the series of numbers generated from 4n+2 where n = zero or any positive integer (ie, n = 0, 1, 2, etc.). A circle mnemonic predicts orbital energies for conjugated rings. Name the following compounds:.
(Theochem) 303 (1994) 2-286 285 Fig. (f) Non-aromatic as drawn, but if H- were removed would give an aromatic cation. Isn't a mathematician Apr 30 '12 at 12:53 |.
The topological background of the “4 n + 2 rule” is also briefly discussed. Earlier we factored this polynomial by splitting the middle term. 4n+2 = Number of Resonating Electrons.
All the compounds obey the Huckel’s Rule, i.e all the aromatic compounds should have the (4n+2) Pi number of electrons. His rule states that if a cyclic, planar molecule has 4n+2 \(π\) electrons, it is considered aromatic. If is 0 or any positive integer (1, 2, 3,), the rule has been met.
The advantage of this approach is that aromaticity is quite stable to perturbations to the symmetry (benzene rings in e.g. Pyrene is a commonly used counter-example of Hückel's rule. (c) An aromatic system because n=2 in the Huckel 4n+2 rule.
Oila, benzene satisfies Huckel's rule. Whether the ring is "aromatic" or not is of no importance. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931.
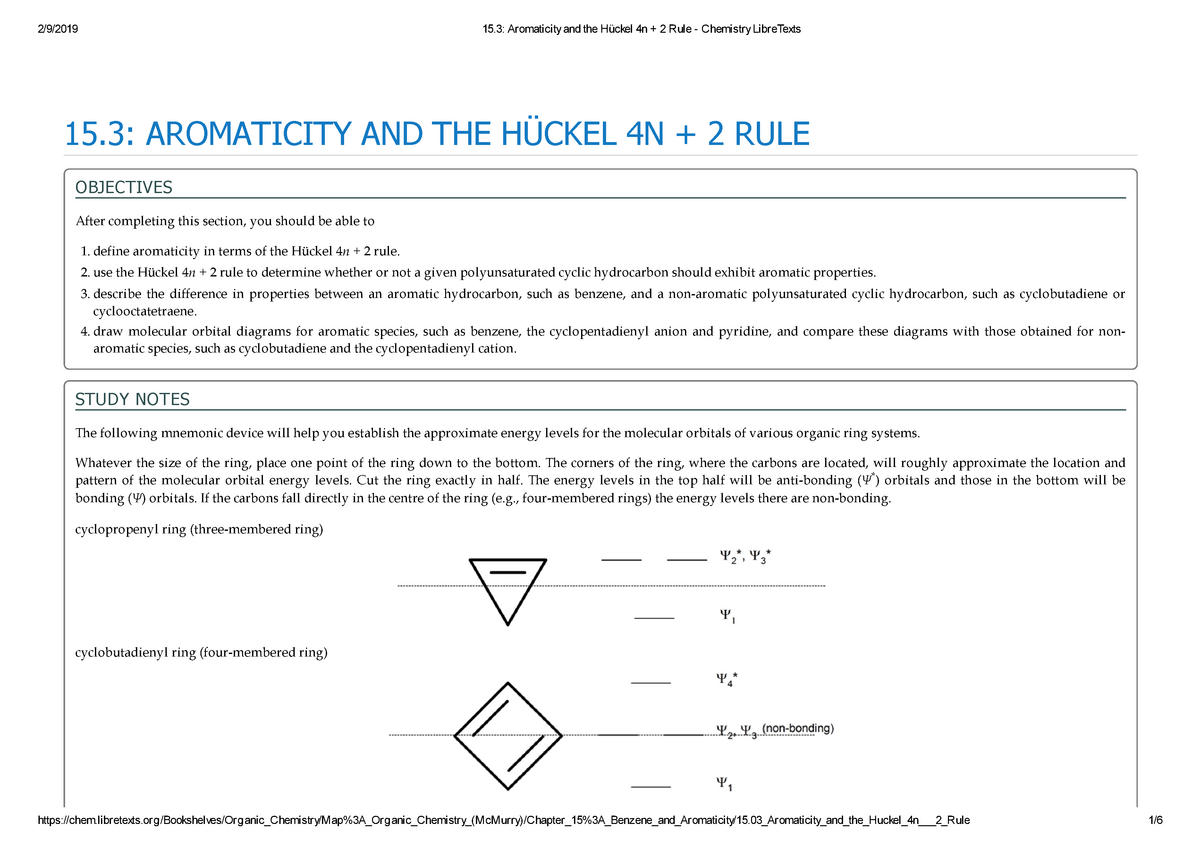
Free math problem solver answers your algebra, geometry, trigonometry, calculus, and statistics homework questions with step-by-step explanations, just like a math tutor. In 1931, German chemist and physicist Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties. 10/10/19 1 15.3 AROMATICITY AND THE HUCKEL 4N + 2 RULE Objectives After completing this section, you should be able to 1.
Using the Huckel 4n + 2 rule determine which of the following compounds are aromatic and which represent conjugated systems:. Definition of huckel's rule wit a lil mix of explanation :. These are depicted in Fig.
For example, benzene has six \(\pi\) electrons:. Huckel’s rule is used to identify the Aromaticity of a compound. Let’s understand it by taking the example of Benzene and Cyclo octa-tetraene.
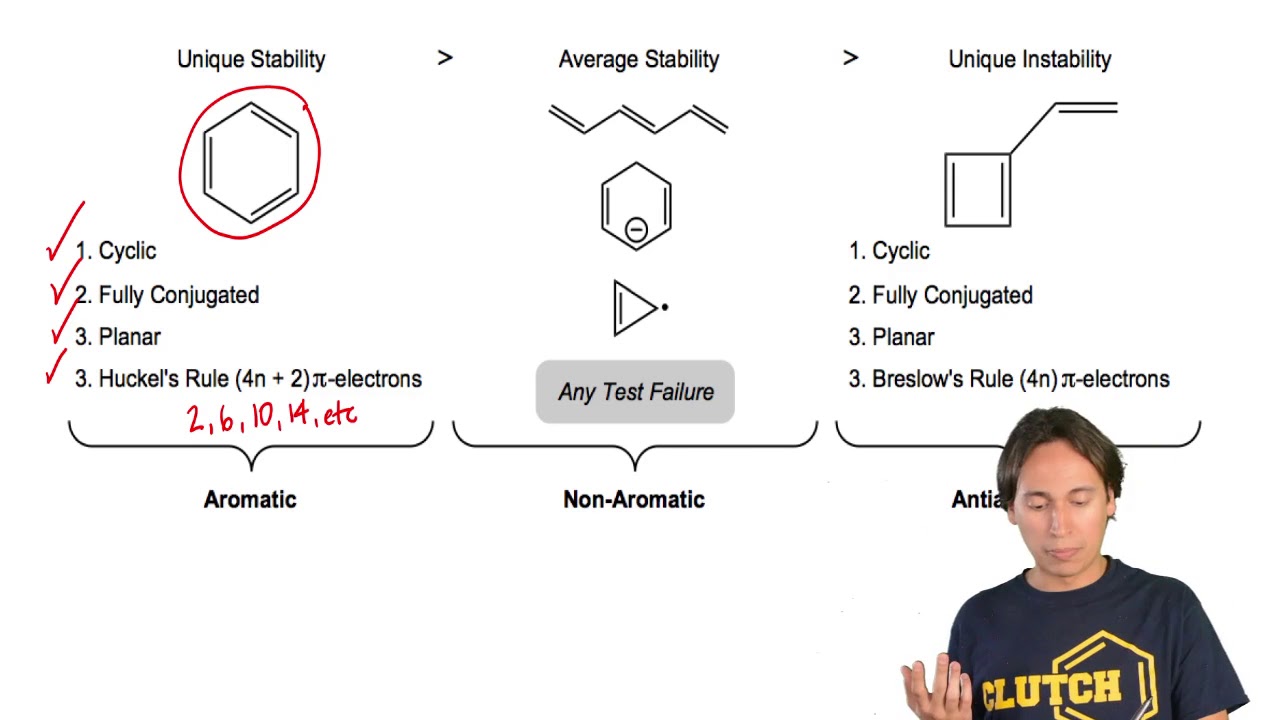
Definitions of 4n 2 rule, synonyms, antonyms, derivatives of 4n 2 rule, analogical dictionary of 4n 2 rule (English). Also known as Hückel's rule. 2, 6, 10, 14 etc.
Another way to put the 4n+2 rule is that if you set 4n+2 equal to the number of electrons in the pi bond and solve for n, you will find that n will be a whole number. Aromaticity plays a major role in the field of biochemistry of all the living structures. Why is cyclooctatetraene not antiaromatic?.
N= 0, 1, 2, 3, 4…etc.). 고리형 평면분자는 휘켈 규칙에 의해 그것의 파이 전자 수가 + (단 이 음 아닌 정수)일 때 방향족성을 띤다. According to Huckel’s rule, all planar aromatic compounds must have 4n+2 pi-electrons where n is an integer (i.e.
If we get a fraction then the molecule DOES NOT obey Huckel’s rule. According to Huckel’s rule, any specie that is cyclic, planar and has (4n+2) delocalized Ï€ electrons will be aromatic and stable. How do you write {eq}(4n)^2 {/eq} without exponents?.
The exponent is used to. Use the Hückel 4 n + 2 rule to determine whether or not a given polyunsaturated cyclic hydrocarbon should exhibit aromatic properties. In order to be aromatic , a molecule must have a certain number of pi electrons (electrons with pi bonds, or lone pairs within p orbitals) within a closed loop of parallel, adjacent p orbitals.
4.1 Find the Vertex of y = 4n 2 +4n+1 Parabolas have a highest or a lowest point called the Vertex. Download my free guide ’10 Secrets to Aci. Therefore n must be a whole number that satisfies this equation 4n+2=x, where x = the number of electrons in the pi bonds.
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. Simply put, Huckel’s rule for aromaticity states that a monocyclic system will be aromatic if there are 4n + 2 delocalised electrons, (n an integer) contained within it. If n equals 1, then 4n+2 equals 6.
(d) A non-aromatic, conjugated 6 p-electron system (e) A non conjugated hydrocarbon. Hückel (4 n + 2) rule. An aromatic compound must have a molecule that contains cyclic cloud of Delocalized π electrons and the π cloud must contain a total of (4n+2) π electrons.
휘켈 규칙(Hückel's rule)은 유기 화학에서 평면 고리 분자가 방향족성을 띠게 되는 조건에 대한 규칙이다. The exponent rule defines that the number of times a value or entity is multiplied with itself. Although its succinct formulation as 4n + 2 was given by Professor William von Eggers Doering.
For example, benzene has 6 π electrons and it satisfies the Hückel’s rule since the n, in this case, is equal to one:. \\begin{align} 4n + 2 &= 6 \\ 4n &= 4 \\ n &= 1 \end{align}\. (g) Non-aromatic as drawn, but has an important resonance structure that is aromatic.
Cyclophanes for example can be bent by up to 30° and still. To apply the 4n+2 rule, first count the number of π electrons in the molecule. In para-quinone you find an alternant system with four bonding piborbitals and four antibonding ones.
Solving 4n 2 +4n+1 = 0 directly. The “4n + 2 rule” is derived analytically at the level of the simple Hückel theory for neutral even-membered chains, their double ions, as well as cations and anions of the odd-membered. Define aromaticity in terms of the Hückel 4 n + 2 rule.
Key IDEAS Hückel 4n + 2 Rule Some molecules with 4n 1 electrons are very unstable by virtue of being antiaromatic. Then, set this number equal to 4n+2 and solve for n. Get more help from Chegg.
You can't ever fit a structure with pendant pi bonds into the "4n+2" rule. Huckel’s Rule for Aromaticity + Time-saving shortcut Need help with Orgo?.
Link Springer Com Content Pdf 10 1007 2f978 94 009 5718 3 2 Pdf
Illustrated Glossary Of Organic Chemistry Term

Solved Why Isn T The Following Molecule Aromatic Select Chegg Com
Q Tbn 3aand9gctn2msa8bylzftgwgj9seyexaol8il Rj7m Fddl7wiqxcdvypy Usqp Cau
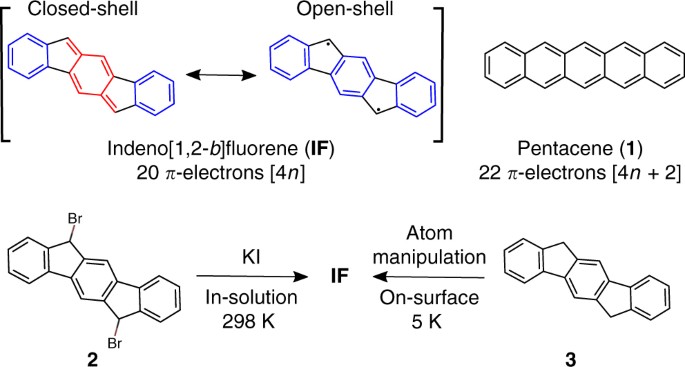
Studying An Antiaromatic Polycyclic Hydrocarbon Adsorbed On Different Surfaces Nature Communications

Huckel S Rule For Aromaticity Time Saving Shortcut Youtube
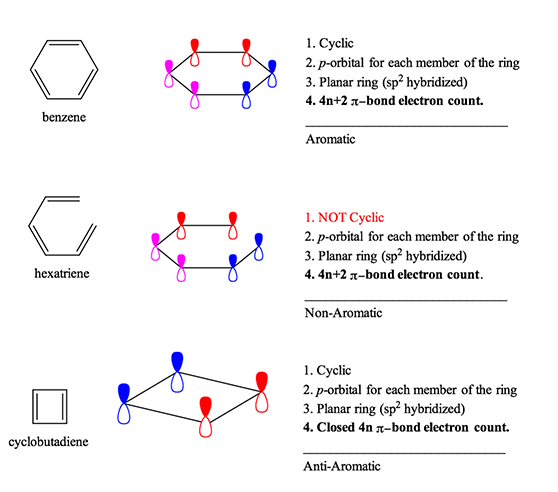
Aromaticity Rules And Definition Organic Chemistry Help
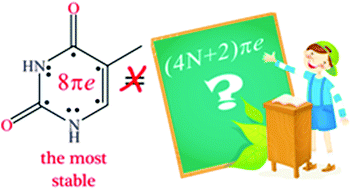
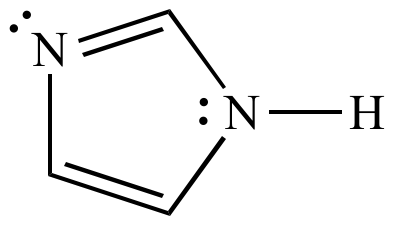
Tautomerisation Of Thymine Acts Against The Huckel 4n 2 Rule The Effect Of Metal Ions And H Bond Complexations On The Electronic Structure Of Thymine Organic Biomolecular Chemistry Rsc Publishing
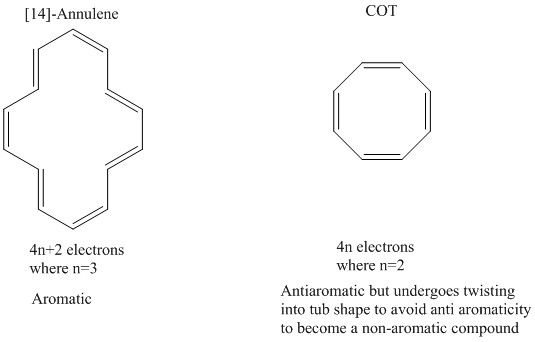
Properties Of Aromatic Compounds Chemistry Revision Site

Aromatic Antiaromatic Or Nonaromatic Huckel S Rule 4n 2 Heterocycles Aromaticity Youtube
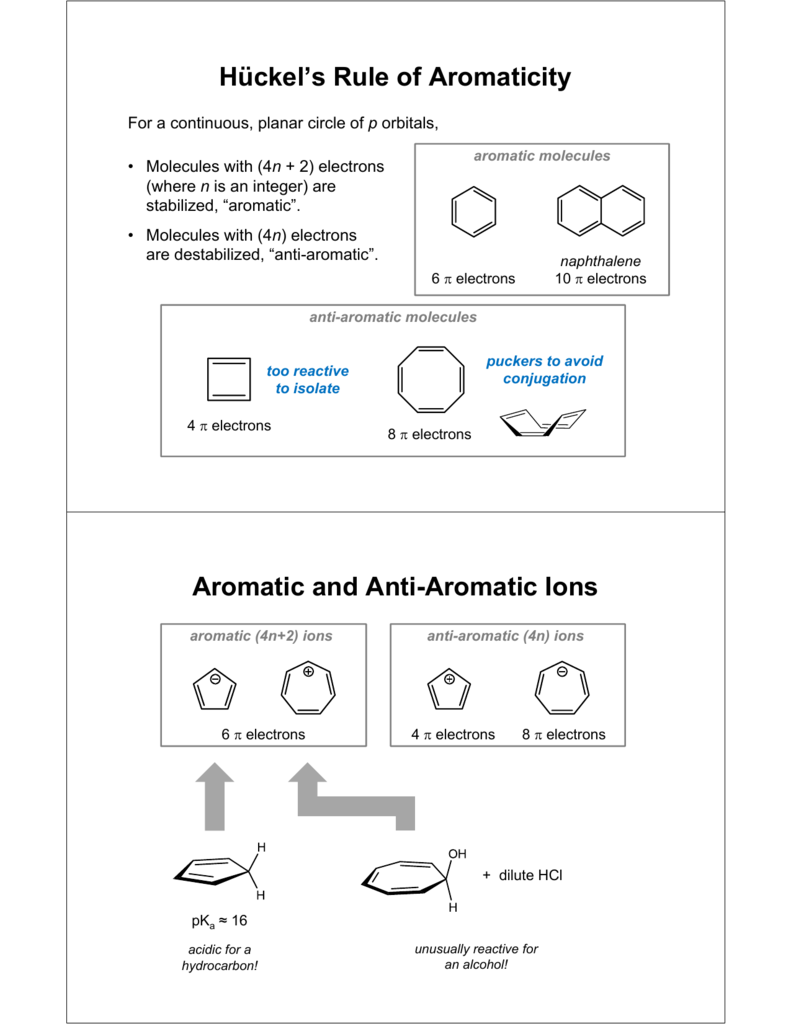
Huckel S Rule Of Aromaticity Aromatic And Anti
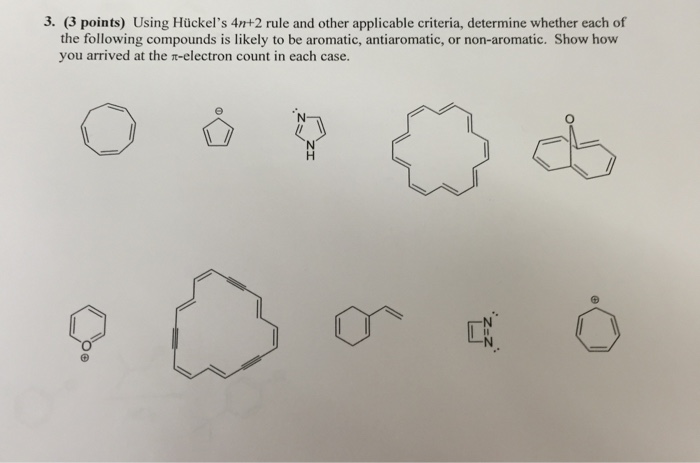
Solved Using Huckel S 4n 2 Rule And Other Applicable Cr Chegg Com
Q Tbn 3aand9gcqpcodx2bi5jpopeynalgtatdsd1mbfuenuzhd39c3vn4zej3xa Usqp Cau
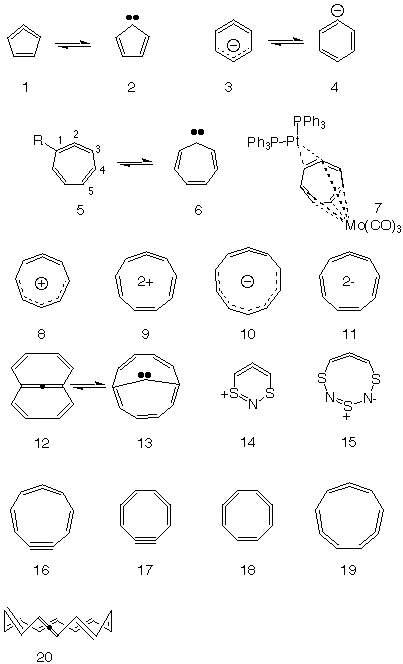
Mobius Aromatics Arising From A C C C Ring Component

Chemistry Huckel S Rule 4n 2 Rule In Order To Be Facebook
2

Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps
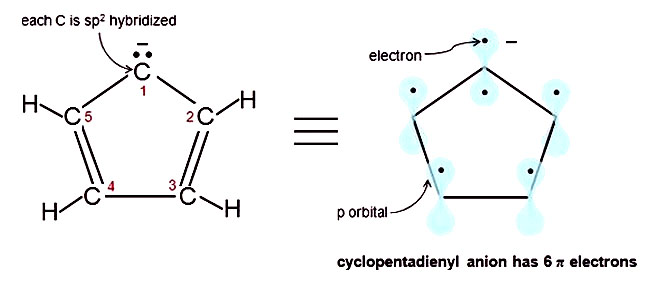
Learn Aromatic 4n 2 Electrons Meaning Concepts Formulas Through Study Material Notes Embibe Com
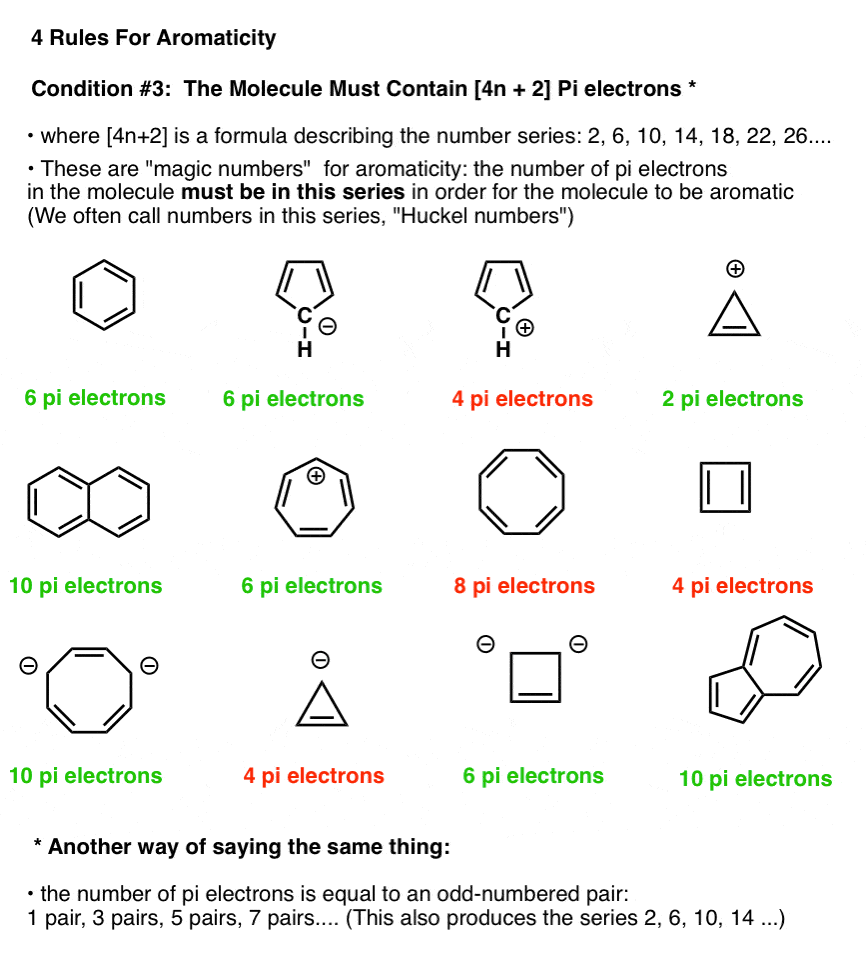
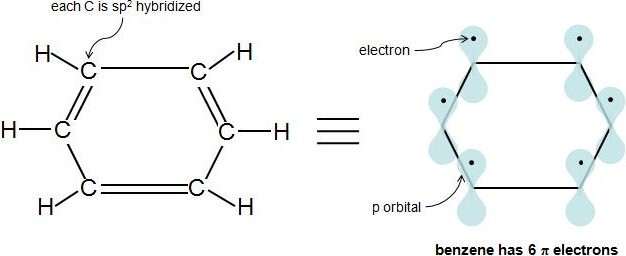
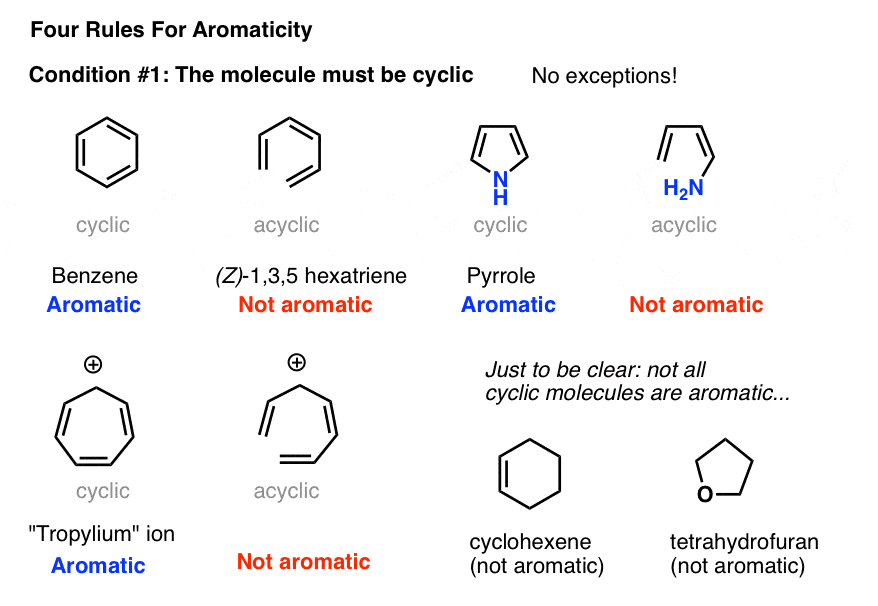
Rules For Aromaticity The 4 Key Factors Master Organic Chemistry
.jpg?revision=1)
15 4 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts

Huckel Rule Or 4n 2 Rule For Aromaticity Examples And Exceptions Youtube
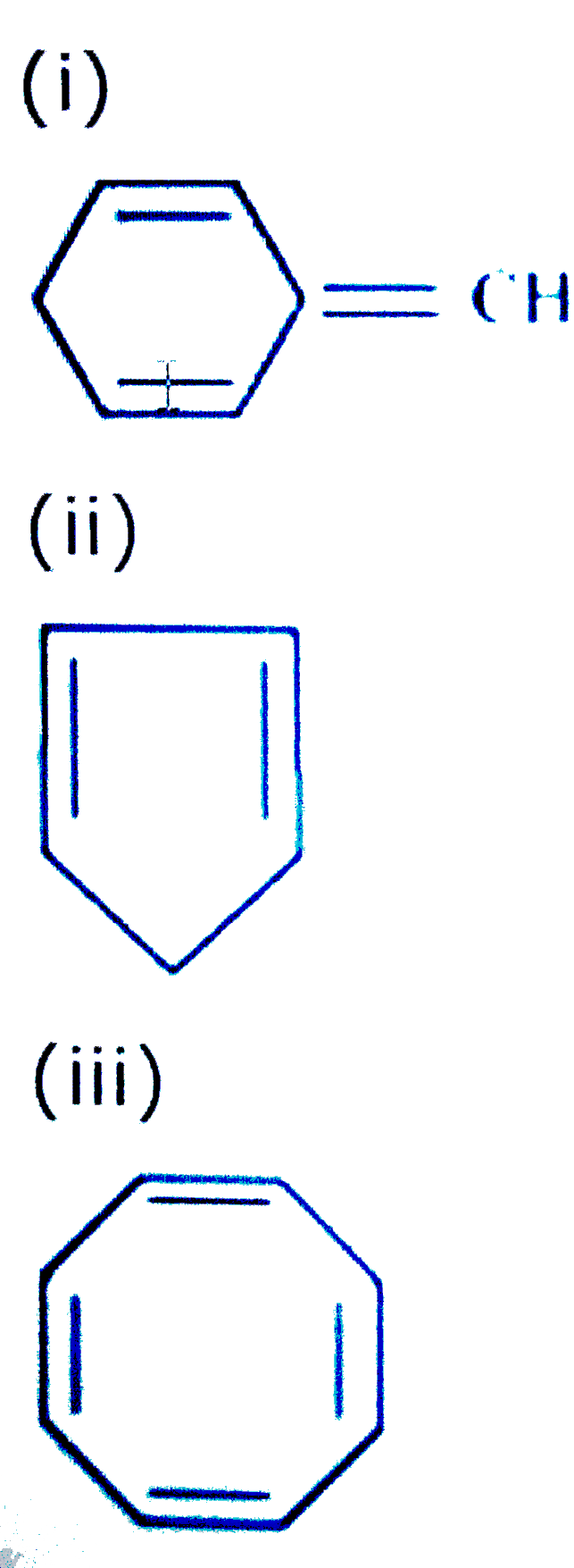
Explain Why The Following Systems Are Not Aromatic Img Src Http

Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps

What Is An Aromatic Compound Definition Example Video Lesson Transcript Study Com
15 Benzene And Aromaticity Ppt Video Online Download

Electrocyclic Reactions
Anti Aromaticity Avoided A Tutorial Example Henry Rzepa S Blog

Chapter 17 Benzene And Aromaticity Ppt Video Online Download
4n 2 Rule Youtube
15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libre Texts Studocu
.jpg?revision=1&size=bestfit&width=317&height=254)
Huckel S Rule Chemistry Libretexts

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps
Pg Chemeasy Huckel S Rule Of Aromaticity And Anti Aromatic Compounds
Huckel S Rule Organic Chemistry Video Clutch Prep

Mobius Aromaticity Wikipedia

Indicate Whether Or Not Each Of The Following Structures Is Considered To Be Aromatic Homeworklib
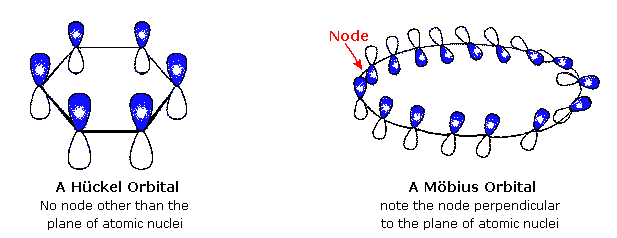
A Mobius 16 Pi Electron System

Huckel S Molecular Orbital Theory Huckel S Rule Extended Huckel
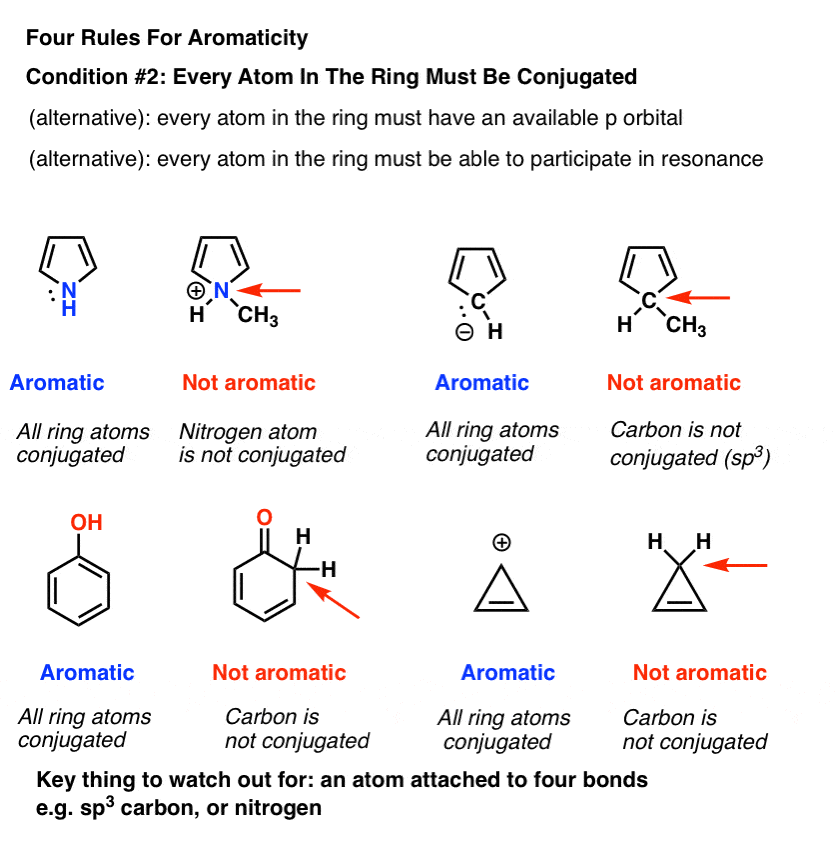
Rules For Aromaticity The 4 Key Factors Master Organic Chemistry
1

13 6 Aromaticity Organic Chemistry Ii

Lecture 17 Chapter 15 Pdf Aromaticity Hckels 4n 2 Rule Only Applicable To Mono Cyclic Systems A Molecule Is Aromatic If 1 Every Atom In The Ring Has Course Hero
Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry
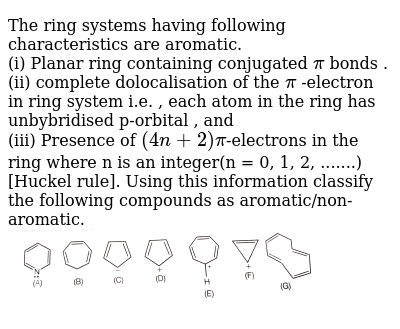
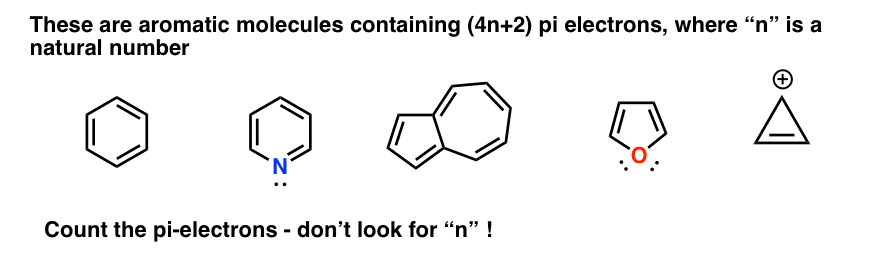
In Huckel S Rule 4n 2 P Electrons What Does N Signifies

109 Aromaticity 2 Huckel S Rule Madoverchemistry Com
Huckel S Rule Organic Chemistry Video Clutch Prep

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps

15 4 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts
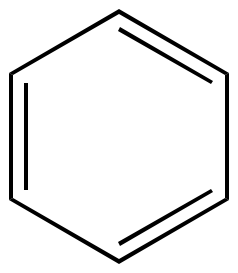
Illustrated Glossary Of Organic Chemistry Term

Aromaticity Tutorial For Cyclic Charged And Heterocyclic Aromatic Compounds
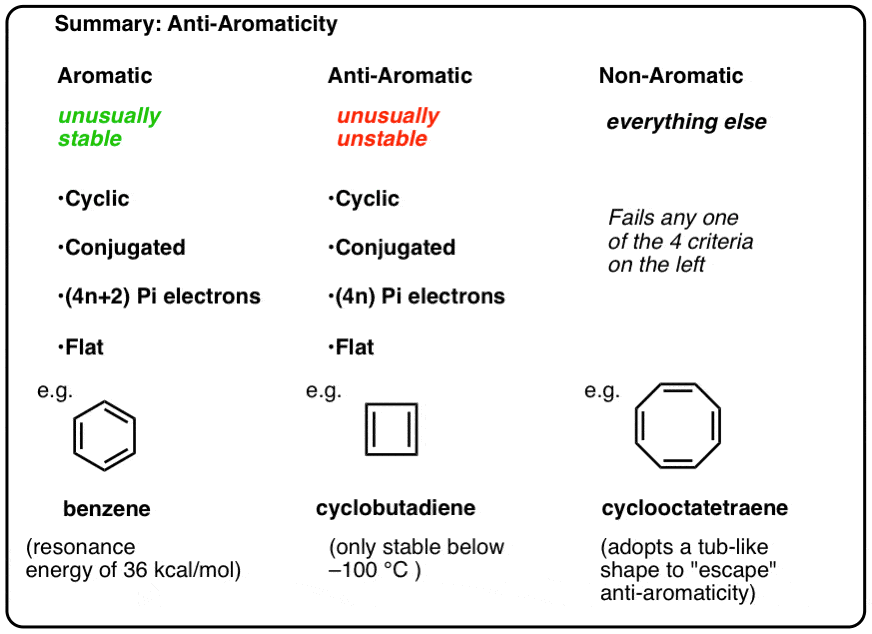
Antiaromaticity And Antiaromatic Compounds Master Organic Chemistry
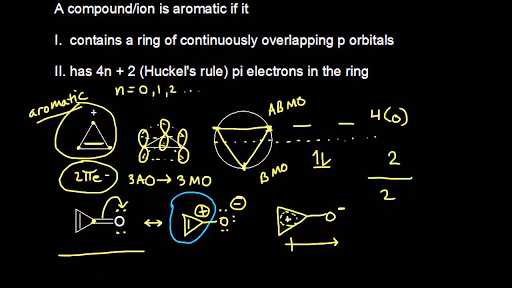
Aromatic Stability Iii Video Khan Academy
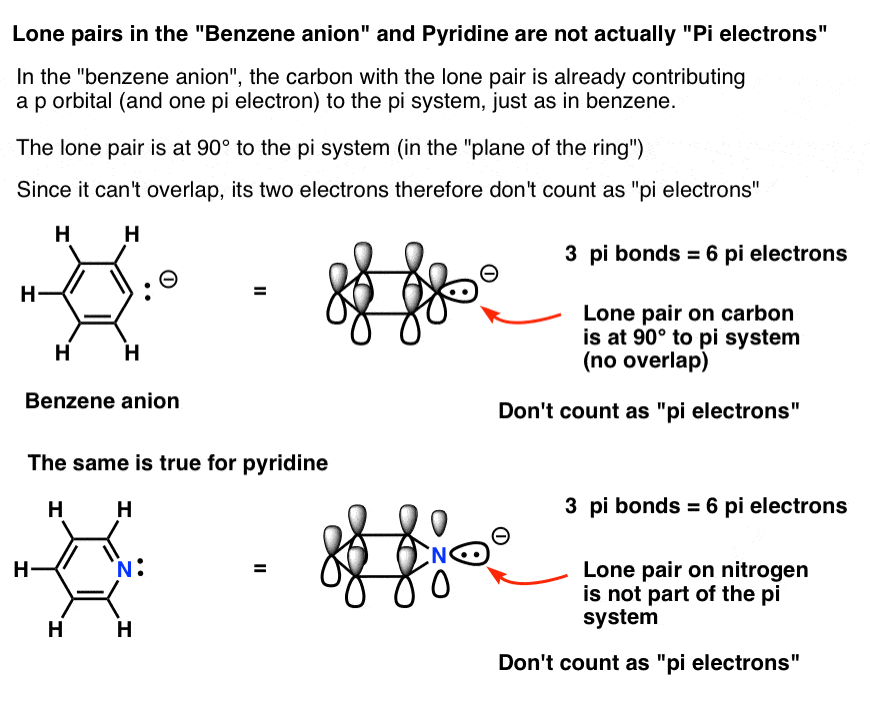
Rules For Aromaticity The 4 Key Factors Master Organic Chemistry
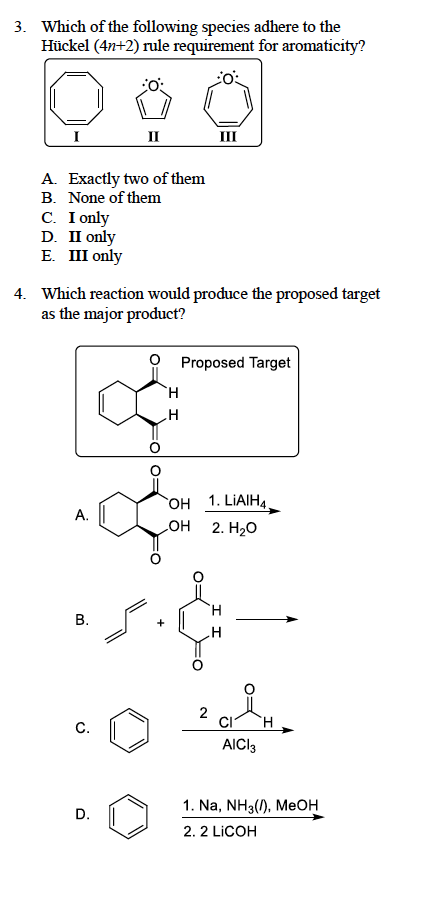
Solved 3 Which Of The Following Species Adhere To The Hu Chegg Com
Rules For Aromaticity The 4 Key Factors Master Organic Chemistry
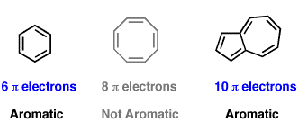
Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry

Aromatic Properties Mcat Question Of The Day
Q Tbn 3aand9gcsd3ah2vdmhe7uydhdx6apoxlogkcmi2fl06j52sg1i2jna0doq Usqp Cau
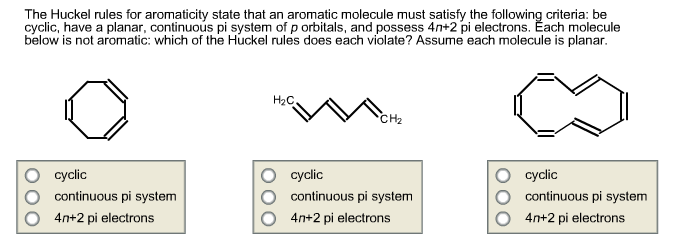
Solved The Huckel Rules For Aromaticity State That An Aro Chegg Com

The Criteria For Aromaticity Mcc Organic Chemistry

The Concept Of Aromaticity Chempapy

Aromatic Compounds And Huckel S Rule Youtube

Indicate Whether Each Structure Is Aromati Clutch Prep

Aromatic Compounds Ppt Video Online Download
The Problem With Pyrene Michael J S Dewar To The Rescue
Aromatic Stability I Video Khan Academy
Chapter 15 Benzene And Aromaticity Ppt Download
Benzene And Aromaticity Ppt Video Online Download
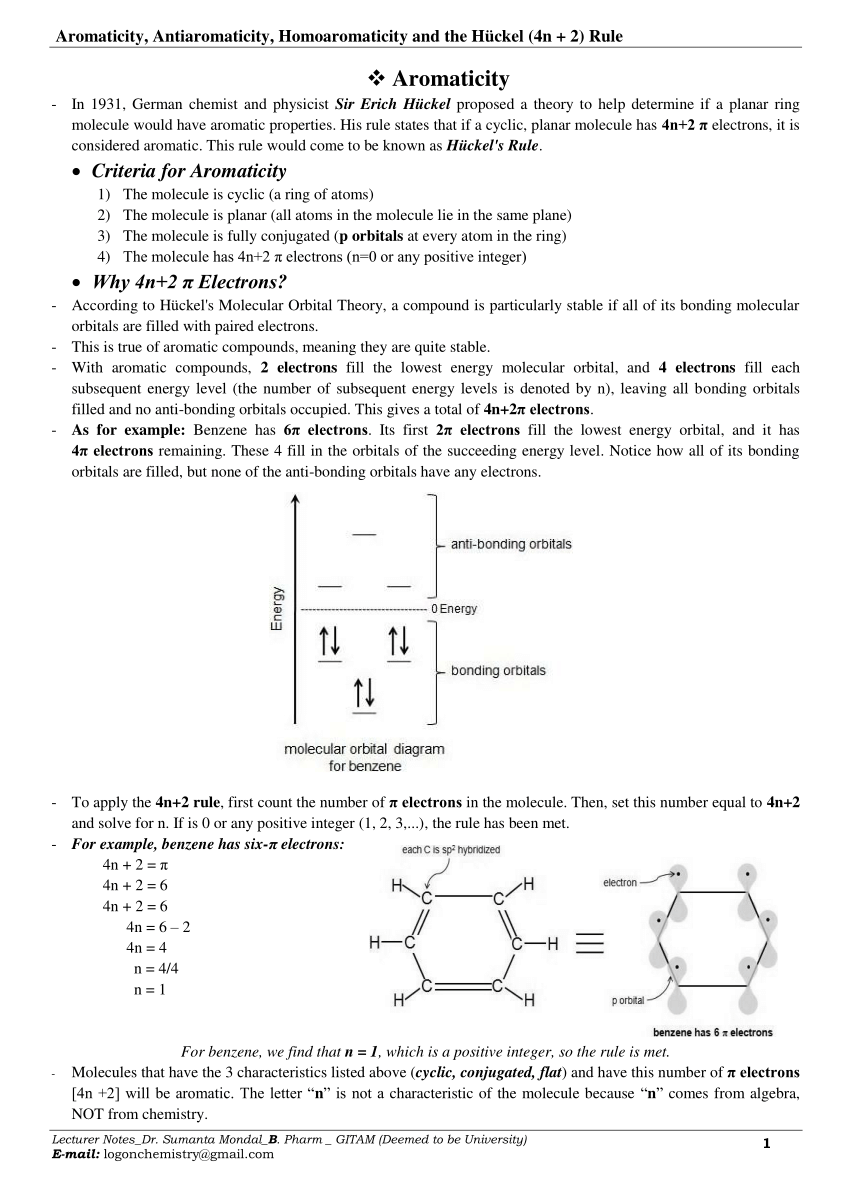
Pdf Aromaticity Antiaromaticity Homoaromaticity And The Huckel 4n 2 Rule
Www Mdpi Com 14 3049 25 3 711 Pdf
Illustrated Glossary Of Organic Chemistry Term
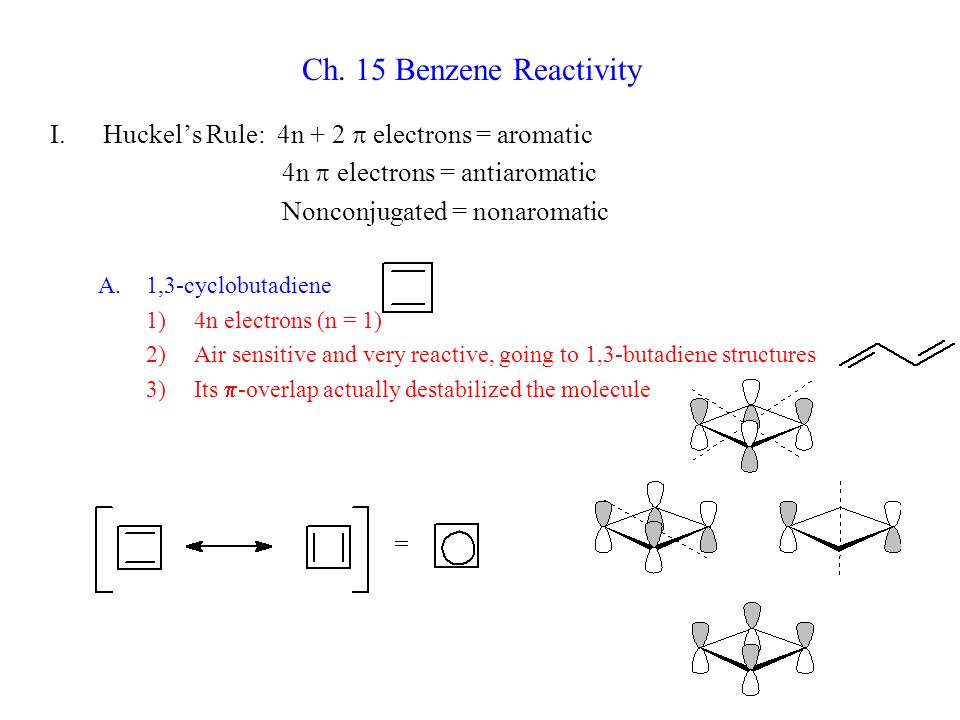
Ch 15 Benzene Reactivity Huckel S Rule 4n 2 P Electrons Aromatic Ppt Video Online Download
2
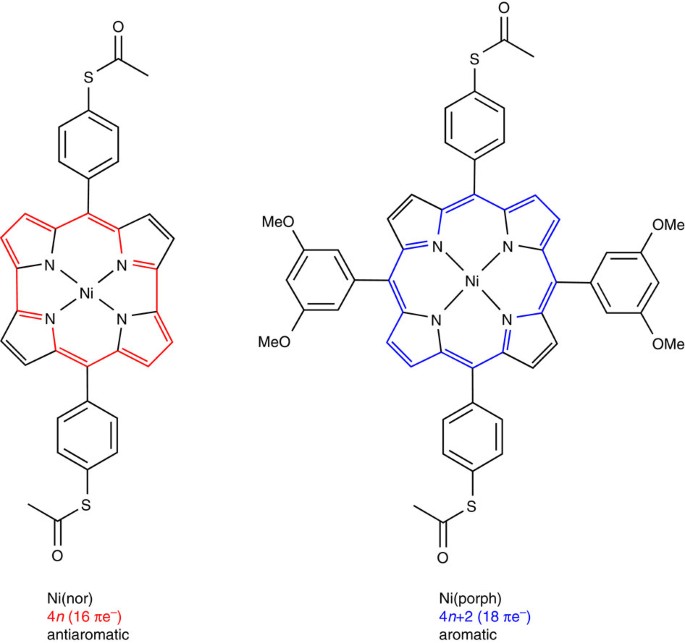
Highly Conducting Molecular Circuits Based On Antiaromaticity Nature Communications
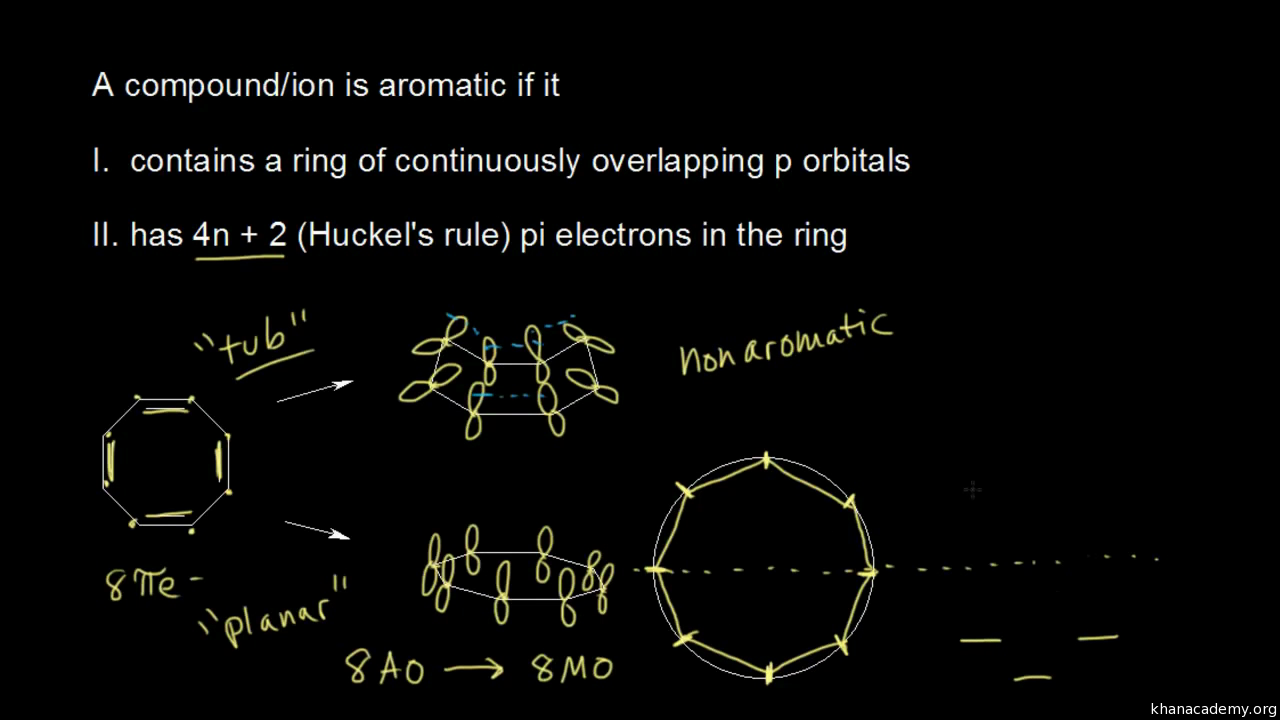
Aromatic Stability Ii Video Khan Academy
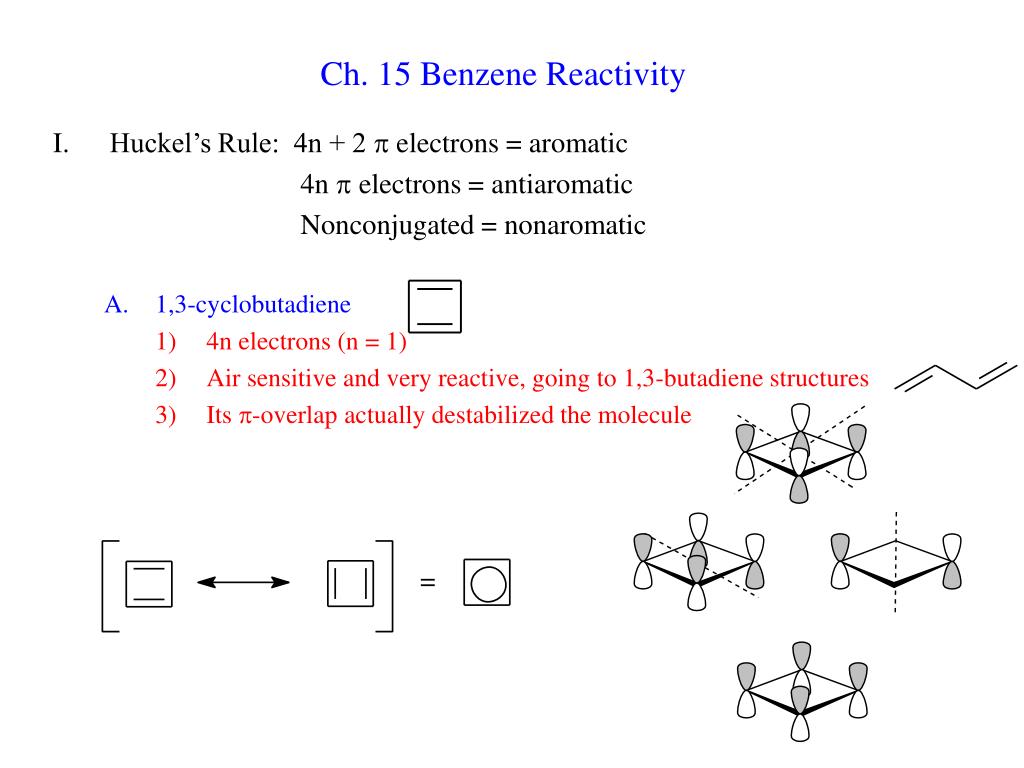
Ppt Ch 15 Benzene Reactivity Powerpoint Presentation Free Download Id

Aromatic Antiaromatic Or Non Aromatic 13 Worked Examples
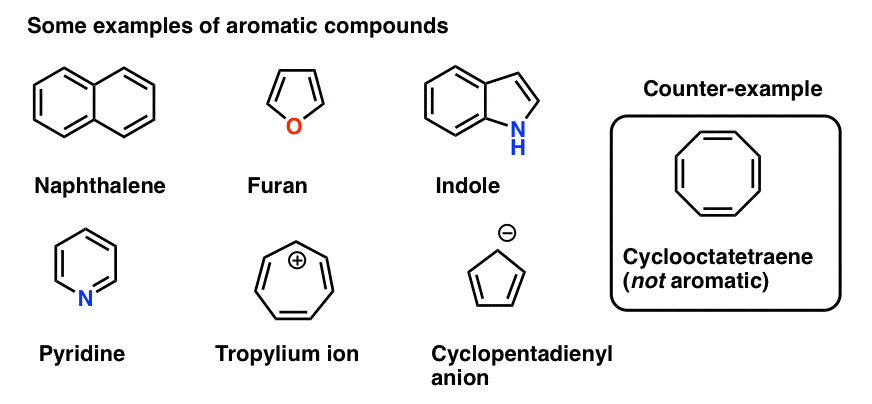
Rules For Aromaticity The 4 Key Factors Master Organic Chemistry
Http Accounts Smccd Edu Batesa Chem235 238 Notes Ch17 Aromatics Print Pdf
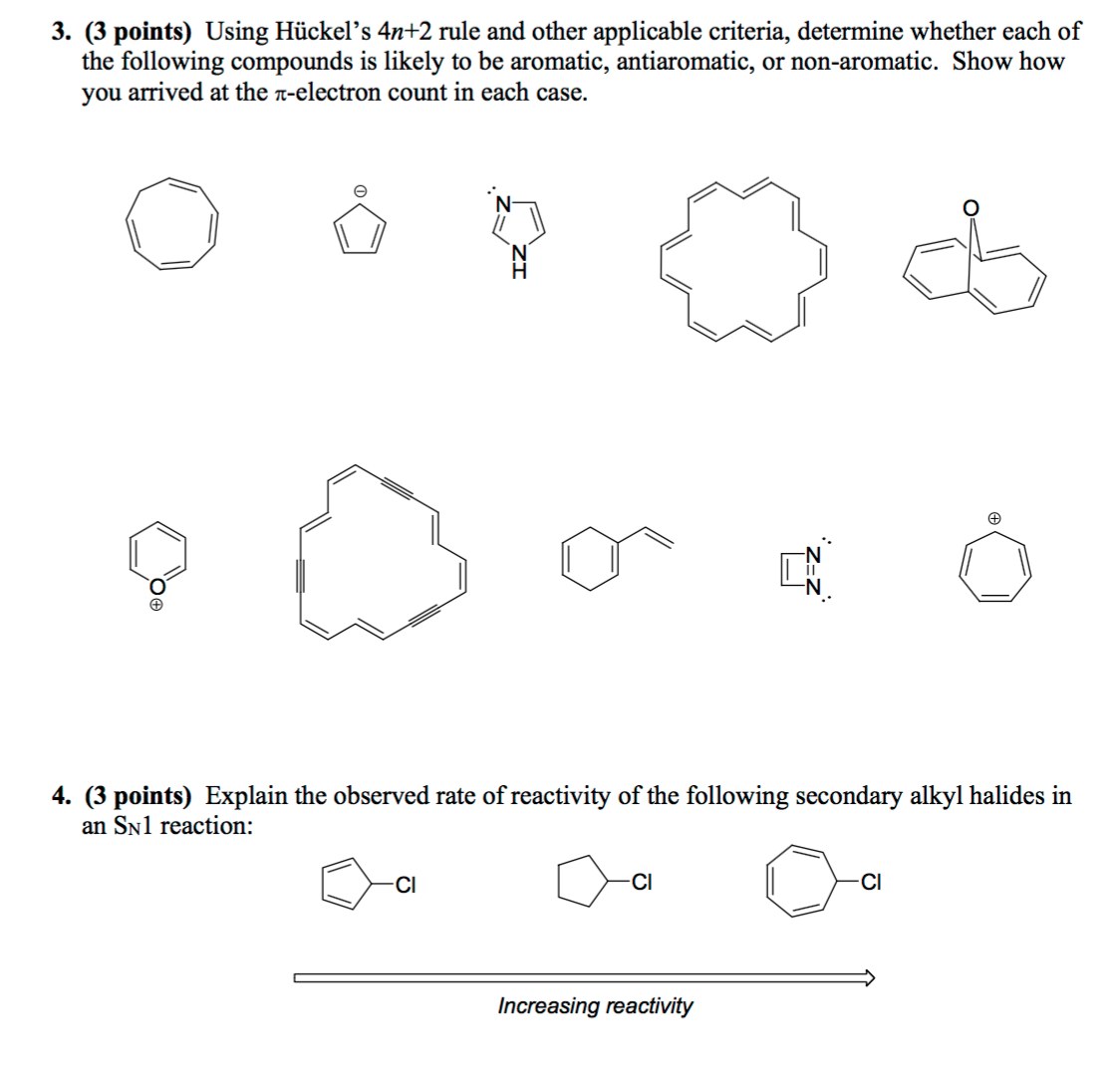
Solved Using Hu Ckel S 4n 2 Rule And Other Applicable Cri Chegg Com
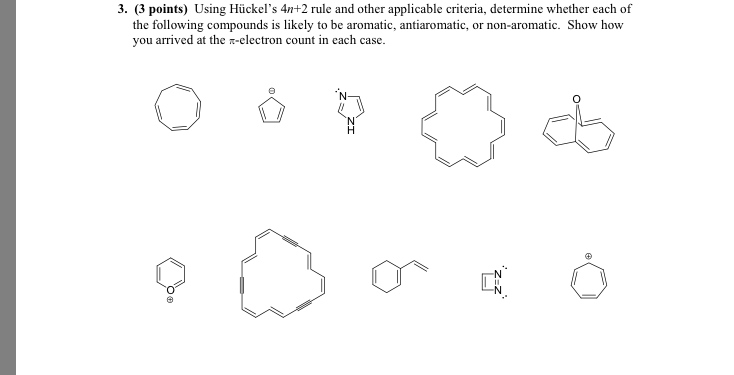
Solved Using Huckel S 4n 2 Rule And Other Applicable Cr Chegg Com

A Disrotatory 4n 2 Electron Anti Aromatic Mobius Transition State For A Thermal Electrocyclic Reaction Henry Rzepa S Blog

13 6 Aromaticity Organic Chemistry Ii
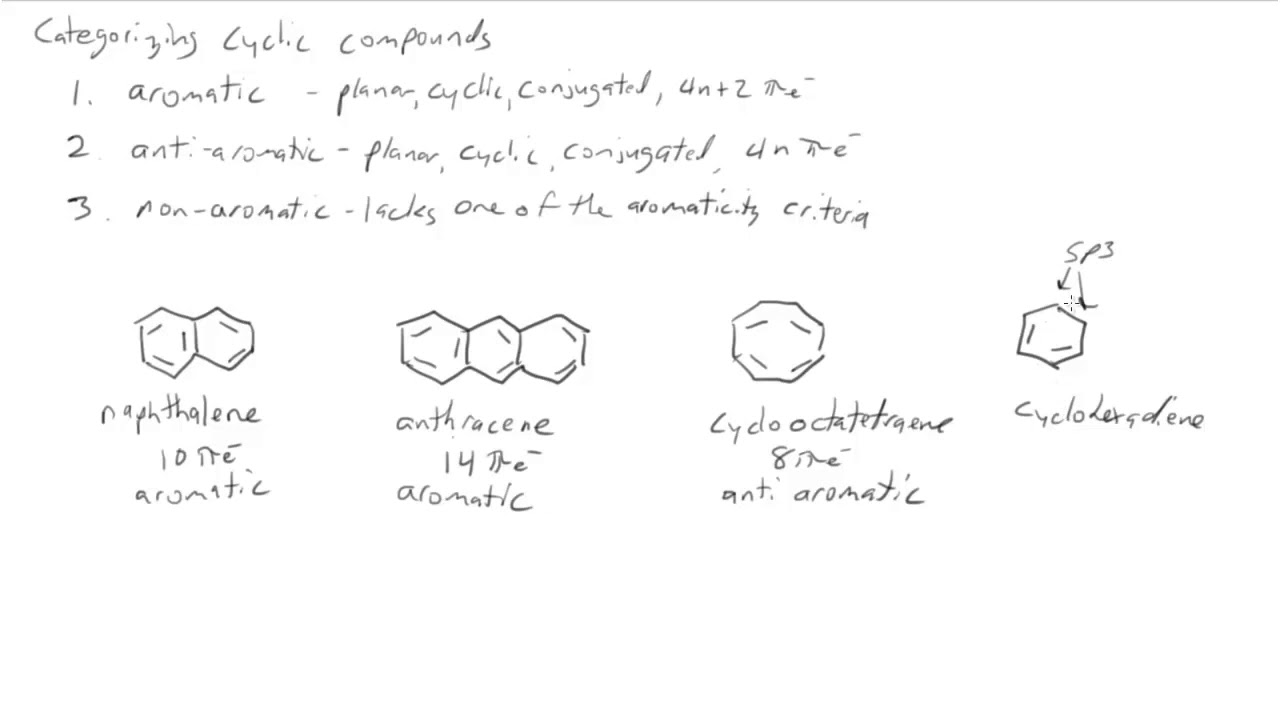
13 7 The Criteria For Aromaticity Huckel S Rule Chemistry Libretexts

Aromaticity And Huckels Rule Powerpoint Slides
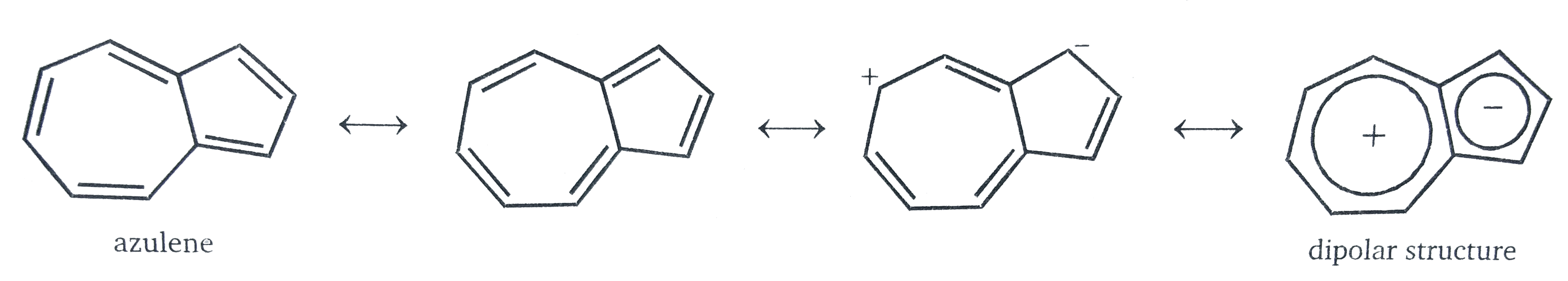
Which Compound Posses Highest Dipole Moment
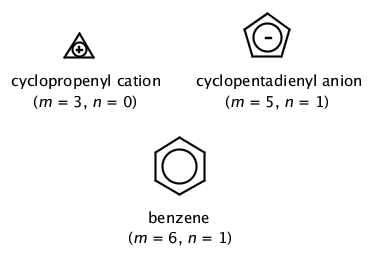
Iupac Huckel 4 Em N Em 2 Rule H

Aromatic Antiaromatic Or Nonaromatic Huckel S Rule 4n 2 Heterocycles Aromaticity Youtube

Quantum Chemistry 14 4 Huckel S Rule Youtube

The Criteria For Aromaticity Mcc Organic Chemistry

Pdf Analytical Derivation Of The Huckel 4 N 2 Rule

Solved What Is The Meaning Of N In 4n 2 Aromaticity R Chegg Com
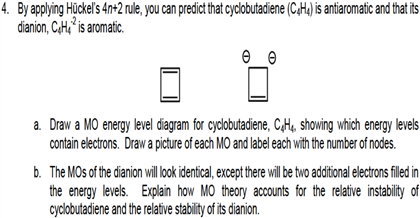
By Applying Huckel S 4n 2 Rule You Can Predict Th Chegg Com
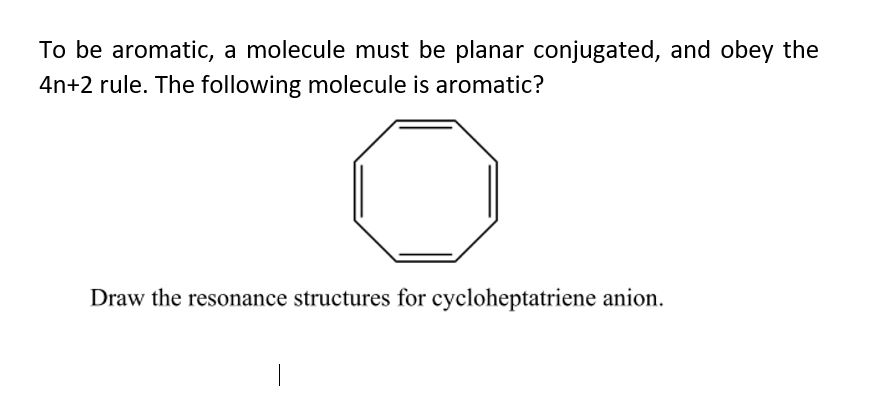
Ywil1z1ntlkcym
Organic Chemistry On Line

15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts Aromaticity Covalent Bond
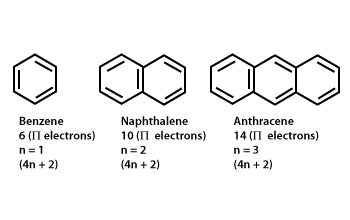
Aromaticity
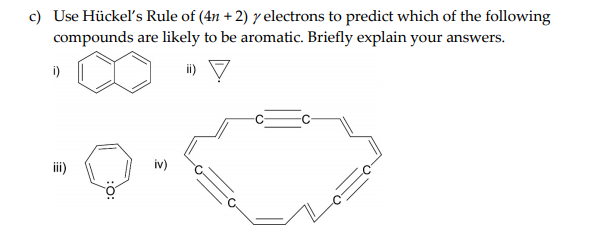
Solved Use Huckel S Rule Of 4n 2 Y Electrons To Predi Chegg Com



